What Is Dysport? An Ultimate Guide to Neuromodulators for Cosmetic Use
Introduction to Neuromodulators/Neurotoxins
The International Neuromodulation Society (2018) defines neuromodulation as “the alteration—or modulation—of nerve activity by delivering electrical or pharmaceutical agents directly to a target area” (para. 5). In cosmetic medicine, practitioners use botulinum toxins to achieve neuromodulation. Botulinum toxins come in seven types, labeled A through G, but only types A and B are currently used in medical treatments (Bach & Simman, 2022). This post primarily refers to botulinum toxin as BoTN or Dysport, aiming to provide an in-depth overview for current patients and those interested in the treatment, though it is not a treatment guide.
BoTN was initially used to treat strabismus (commonly referred to as crossed eyes) in the 1970s, receiving FDA approval for this use by 1989, along with approvals for blepharospasm (eye twitching) and hemifacial spasms (twitching of one side of the face) (Bach & Simman, 2022).
Its effectiveness for treating wrinkles was first noted in 1987 by ophthalmologist Jean Carruthers. After administering BoTN to a patient to treat eye twitching, the patient noticed a reduction in wrinkles around the eyes and asked if it could be used on her forehead. A future publication by her husband, dermatologist Alastair Carruthers, set the stage for the future of BoTN as a cosmetic medicine (Park & Ahn, 2021). As per the American Society of Plastic Surgeons (2023), over “8.7 million aesthetic treatments were performed using BoTN in 2022 alone, making it the world’s most popular minimally invasive cosmetic procedure” (p. 10).
What is Dysport and How Does It Work?
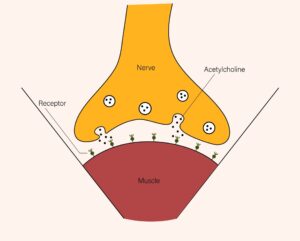
In the United States, there are currently six available formulations of botulinum toxin (BoTN): onabotulinumtoxinA (Botox), abobotulinumtoxinA (Dysport), incobotulinumtoxinA (Xeomin), prabotulinumtoxinA-xvfs (Jeuveau), daxibotulinumtoxin-A-Ianm (Daxxify), and rimabotulinumtoxinB (Myobloc) (Bach & Simman, 2022; Truong et al., 2023). BoTN works by interrupting the communication between nerves and muscles. The brain and spinal cord send nerve signals, called action potentials, which travel to a connection point between the nerve and muscle, known as the neuromuscular junction. The pathway varies slightly depending on whether the movement is voluntary or involuntary. This triggers a series of events that cause the release of acetylcholine and its binding to the muscle fiber, causing movement.
BoTN interrupts the series of events that release acetylcholine, and since it can then not bind with the muscle, contraction cannot occur. BoTN-A and BoTN-B achieve this by targeting different proteins required for acetylcholine release, with BoTN-A targeting SNAP-25 and BoTN-B targeting VAMP (Bach & Simman, 2022; Choudhury et al., 2021; Ong & Sherris, 2019).
Figure 1
Acetylcholine Release
What are neuromodulators used for?
BoTN’s ability to block muscle contraction enables it to treat a variety of medical issues, with researching still discovering new uses. In this post, we will be focusing on cosmetic treatments, but some other examples include:
- Ophthalmology (eye twitching, crossed eyes).
- Neurology (cervical dystonia, tremors, tics, spasticity, spasms).
- Orthopedics (cerebral palsy, multiple sclerosis).
- Gastroenterology (swallowing disorders, throat spasms, delayed gastric emptying).
- Urology (overactive bladder, incontinence).
- Rheumatology (Raynaud’s phenomenon) (Bach & Simman, 2022).
It has also shown promise as a potential treatment for depression, hypertrophic and keloid scarring, facial flushing, oily skin, rosacea, alopecia, and psoriasis (Guida et al., 2018; Lewandowski et al., 2022; Park & Ahn, 2021; Schulze et al., 2021).
- Botox is currently approved for the treatment of frown lines, forehead lines, and crow’s feet;
- Dysport is approved for frown lines, cervical dystonia, and spasticity;
- Xeomin for Xeomin chronic sialorrhea, limb spasticity, cervical dystonia, blepharospasm, and frown lines;
- and Jeuveau solely for frown lines (AbbVie, 2023; Evolus, 2023; Galderma, 2023; Merz, 2023).
Practitioners commonly use all four medications off-label for other cosmetic purposes. Myobloc, the only currently approved BoTN-B medication, has FDA approval for cervical dystonia and chronic sialorrhea (Solstice Neurosciences, 2019). Although it is occasionally used for cosmetic treatments, this is much less common.
For aesthetic purposes, practitioners primarily use BoTN to treat dynamic wrinkles (Witmanowski & Błochowiak, 2019). Dynamic wrinkles occur with movement, whereas static wrinkles remain on the face at rest.
Since BoTN works by limiting movement, it is not ideal for treating static lines, though it can help prevent these from forming or becoming deeper. It is also used to slim muscles, often for a feminizing effect. BoTN has a different function from dermal fillers, which are used to provide volume and structure.
What are the differences between the various neuromodulators?
There are several differences between the formulations of botulinum toxin medications, notably that Botox, Dysport, Xeomin and Jeuveau are BoTN-A formulations, while Myobloc is a BoTN-B formulation. As mentioned previously, BoTN-A and BoTN-B block the release of acetylcholine to prevent muscle contraction through different methods, with BoTN-A targeting SNAP-25 proteins and BoTN-B targeting VAMP proteins.
Manufacturers currently sell Botox, Dysport, Xeomin, and Jeuveau as powder compounds that must be reconstituted with saline before injection. In contrast, they sell Myobloc as a ready-to-use solution (Choudhury et al., 2021). While this could appear advantageous for Myobloc due to the potential risk of error with reconstitution, the reconstitution portion of treatment is such a basic aspect of the procedure that any concerns about this step should warrant further concern of the provider’s experience or necessary oversight. Myobloc is rarely used for cosmetic purposes, so we will not be examining it in detail in this post.
Below, we’ll explore the differences between BoTN-A products in greater depth.
Botox (onabotulinumtoxinA)
Botox was the first cosmetic BoTN product to market. Vials come in volumes of 50 or 100 units. The onset of action (how long it takes to start showing an effect) is usually 3 to 5 days but can be up to 2 weeks. The effects usually last 3 to 6 months, with a normal duration of 3 to 4 months. It is typically the most expensive when compared to other brands.
Figure 2
Botox Cosmetic

Dysport (abobotulinumtoxinA)
Dysport was the second cosmetic BoTN medication and comes in vials of 300 to 500 units. It is generally the least expensive but requires more units than Botox. The effects of Dysport begin within 24 hours (though it still takes time for peak effect) and last 3 to 6 months, with a typical duration of 4 months (Bach & Simman, 2022; Salame et al., 2023). Dysport contains more active ingredient than Botox and Xeomin (Shtefan et al., 2022).
Figure 2
Dysport

Xeomin (incobotulinumtoxinA)
Xeomin is available in 100-unit vials and begins to take effect in 5 to 7 days. Effects also last 3 to 6 months (Bach & Simman, 2022). The main difference with Xeomin’s formulation is the lack of neurotoxin-associated proteins that are seen in other products. These associated proteins are non-active in the other brand’s formulations but could make the development of antibodies more likely. This occurrence could render treatment less effective (Park et al., 2020). We will explain this in further detail later in the “risks and side effects” portion of this article. Another benefit of Xeomin is that it can be stored safely at room temperature (Salame et al., 2023).
Jeuveau (prabotulinumtoxinA-xvfs)
Jeuveau is a newer form of BoTN-A product that has shown similar efficacy to Botox but at a lower cost (Hanna & Pon, 2020). It is available in 100 unit vials (Evolus, 2023).
Daxxify (daxibotulinumtoxin-A-Ianm)
Daxxiy is an even more recent formulation that received FDA approval in 2023. It was anticipated to be a longer-lasting product. It features a unique protein that was hypothesized to bind longer and more tightly to the nerve terminal, increasing the duration of the treatment to 6 months and reducing the potential spread of the effect (Solish et al., 2021). Like Xeomin, it can also be stored at room temperature and comes in 100-unit vials (Salame et al., 2023). However, we and our colleagues are skeptical about the promised duration and have not found this to be the case in practice. Since the product is so new, research is still scarce.
Understanding Units of Neuromodulators
It is important to note that one unit of one product does not equal one unit of another product, as there is no standard measurement for one “unit” and each manufacturer determines how many “units” they label their vials as (Solish et al., 2021). It is generally believed that 1 unit of Botox is equal to 1 unit of Xeomin, 1 unit of Jeuveau, 2.5 to 3 units of Dysport, and 1.5 to 2 units of Daxxify ( Bellows & Jankovic, 2019; Choudhury et al., 2021; Gadarowski et al., 2021; Salame et al., 2024).
How much does BoTN cost?
For the sake of convenience, our office uses Botox units as a method of conversion and billing for patients, with 1 unit of neuromodulator meaning 1 unit of Botox, Xeomin, and Jeuveau or 3 units of Dysport. We find this less confusing for new patients than utilizing a separate counting system for different brands, especially if they were previously using a different product. When discussing “units” here and in the next section, we are referring to “Botox units.” Please note that if you are seeking care from another provider using Dysport, Daxxify, or Myobloc, they may be using a different method of measurement.
The cost for BoTN depends on the product being used, the city you are in, and the provider’s experience. Currently, at Allure Aesthetics, we charge $12 per unit.
What areas can be treated with Dysport, and how many units do I need?
Aesthetic offices commonly use Dysport for the following areas, requiring approximately the listed number of units. Please note that units vary depending on goals, age, and individual anatomy:
Upper Face
- Forehead lines (Frontalis): The forehead is often treated to reduce the lines that appear when lifting the brows. 8–24 units are generally required, spread over 4 to 12 areas of the forehead.
Figure 3
Forehead Lines Before & After
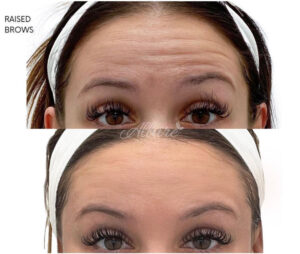
- Brow lift (Glabellar Complex/Orbicularis Oculi): Treatment of the muscles in the forehead and/or between the brows can adjust the arch of the brows.
- Frown lines (Glabellar Complex): This was the first FDA-approved cosmetic treatment for Botox. Approximately 15 to 60 units can be used to relax the muscles between the brows to reduce and prevent wrinkles.
Figure 4
Frown Lines Before & After
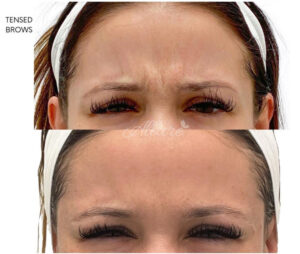
- Crows feet (Lateral Orbit): The muscles on the side of the eyes can be injected to relax them and prevent the “scrunching” appearance of crows feet. This typically requires 4 to 25 units per side.
Middle Face
Figure 5
Crow’s Feet Before & After
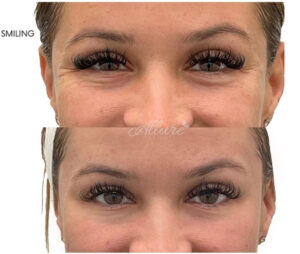
- Bunny lines (Nasal Sidewalls): The muscles of the nose can be treated to also prevent the “scrunching” appearance on the sides of the nose that appears when smiling. 4 to 8 units are typically required (Ong & Sherris, 2019).
Figure 6
Bunny Lines Before & After
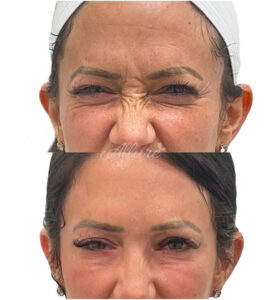
- Nose tip lift (Depressor Septi): In some patients, the tip of the nose may droop downward, which can also make the bridge of the nose appear to have a larger bump. Dysport can help to relax the muscles and elevate the tip of the nose (Yi et al., 2023).
Lower Face
- Smoker’s lines (Vertical Perioral Lines): Dysport can help alleviate wrinkles around the mouth. It is important to reiterate that Dysport mainly helps with dynamic lines—that is, lines that appear with movement. The treatment will not help static lines—lines that remain at rest. Approximately 3 to 7 units are used for this area.
- Gummy smile: A Gummy smile is defined as 2 mm or more of gums showing when smiling (Ong & Sherris, 2019). The upper lip muscle can be relaxed to prevent it from pulling up as far, reducing the amount of gums that show. About 2 to 20 units are required.
- Lip flip: A newer treatment that has become popular on social media, a lip flip works in a similar way to a gummy smile treatment. This works best when your top lip “curls under” when smiling, making it appear small. This treatment can relax the muscles that do this, allowing it to appear larger when smiling. About 2 to 10 units are required.
- Marionette lines (Melomental Folds): The melomental folds, or marionette lines, are lines that can form between the corners of the mouth and the jaw when frowning. Dysport can be used to relax these muscles and reduce the appearance of these lines. Approximately 4 units are injected on each side for this treatment (Ong & Sherris, 2019).
- Masseters: Masseter treatments work a bit differently than those previously listed. Rather than reducing muscle contraction to prevent wrinkles, Dysport can be injected into the masseters (the muscles on the side of the jaw), to relax them and reduce their size. This can reduce the squareness of the face or wideness of the jaw, producing a slimming effect. About 15 to 20 units are usually required on each side (Park & Ahn, 2021; Ong & Sherris, 2019)
Neck
- Nefertiti lift: Named after Nefertiti, the Egyptian queen known for her long neck and defined jawline, this treatment intends to provide a lifting and defining effect to both areas. Dysport is used to relax the muscles that pull down on the jaw and neck. Approximately 20 units are injected on each side (Ong & Sherris, 2019).
- Platysmal bands: The platysmal bands are the long muscles that run down the front of the neck. As we age and our skin thins, these muscles can protrude and become more prominent. They can be injected with Dysport to relax them. 20 to 50 units are usually needed.
- Trapezius (Trap Tox/Barbie Tox): With this newly popularized treatment, Dysport is injected into the trapezius muscles on the back of the neck. Similar to masseter treatment, it is intended to relax them and reduce their size, making the neck appear longer and thinner.
Other Medical Treatments
The treatments below are more medical in nature but are still often offered at medical spas and cosmetic medicine practices. It is important to note that many of these offices do not accept health insurance, though some insurance companies may reimburse for these treatments.
- Migraines: Although it may seem counterintuitive, studies about BotN’s ability to relieve tension headaches have been mixed. However, they have a proven track record in improving chronic migraines ((Do et al., 2018; Kępczyńska & Domitrz, 2022). A meta-analysis (research that combines and analyzes the results of multiple experiments) of 17 studies and 3646 patients found that patients experienced 1.56 fewer migraines per month after 3 months and reported an improvement in their life quality (Bruloy et al., 2019).
- TMJ/Bruxism (Teeth Grinding): BoTN can be used for both TMJ (pain and clicking in the jaw) and bruxism (teeth grinding) by relaxing the jaw muscles. It can help to both lessen the severity and reduce the frequency of incidents (Fernández-Núñez et al., 2019).
- Excessive sweating (Hyperhidrosis): Approximately 3% of the population suffers from hyperhidrosis (excessive sweating) (Guida et al., 2018). Similar to its effect on muscle contraction, BoTN’s blocking of acetylcholine also reduces the activity of the sweat glands, improving this issue. It can also last much longer than cosmetic treatments, with durations of up to 6 to 8 months (Nawrocki & Cha, 2020). The treatment reports a high level of satisfaction when compared to other treatments (Guida et al., 2018; Nawrocki & Cha, 2020).
What are the risks and side effects of Dysport treatment?
Reducing the risk of side effects hinges on the expertise of your provider, who not only selects the appropriate dosage but also demonstrates superior injection technique and precision (Kroumpouzos et al., 2021). This ensures optimal results, a longer duration of effect, and better value for your investment. It’s also important to note that while a provider may possess extensive theoretical knowledge, their practical experience could be limited, particularly if they only perform Dysport treatments sporadically. The best provider will have a profound understanding of anatomy and theory, extensive real-world experience, and ongoing practice in administering treatment. Following pre-care and post-care instructions also helps lower the chances of side effects and complications.
The most common adverse effects of Dysport are the effects of the injection itself, including pain, swelling, redness, and bruising (Lee et al., 2020; Witmanowski & Błochowiak, 2019). Pain should be relieved within an hour, and the other effects within a few days. All of these effects can be lessened with pre-care and comfort measures. Our office utilizes ice, numbing creams, lidocaine, and nitrous to manage pain, though pain is typically minimal.
Headaches
Headache is a potential side effect but is usually short-term. This can occur because the muscle first spasms before relaxing, but it can also be attributed to the trauma of the needle or anxiety from the procedure. In the case of Botox, about 9% of those being treated for forehead lines experienced headaches, but this figure is far higher than we’ve experienced in practice. Most headaches pass within a few days, though some reports of long-lasting headaches (2 to 4 weeks) exist (AbbVie, 2023; Witmanowski & Błochowiak, 2019).
Allergic Reactions
An allergic reaction is a rare but potential adverse event that can result in anything from local redness and swelling to overall itching and anaphylactic shock (rash, nausea, vomiting, difficulty breathing). Mild reactions should subside on their own or can be treated with antihistamines, while anaphylaxis is a medical emergency that requires epinephrine treatment (Witmanowski & Błochowiak, 2019). Those with a history of multiple or severe allergies may not be ideal candidates for Dysport treatment and should let their provider know. As Dysport contains lactose, those with an allergy to cow’s milk protein may be better suited using an alternative product such as Botox or Xeomin (Galderma, 2023).
Dry Skin
There have been reports of dry skin after treatment. This is thought to occur due to decreased activity of sweat glands (similar to how treatment helps excessive sweating). Infection is a rare but potential complication that can occur with any procedure that pierces the skin (Witmanowski & Błochowiak, 2019). This would require further treatment to remedy.
Facial Asymmetry and Movement Limitations
Other complications generally consist of facial asymmetries and movement limitations which can occur from too high of a dosage, a spread of effect, or an imbalance of muscle function. These can be corrected with additional treatment to rebalance muscle function in some cases. In other cases, these effects would slowly improve as the effect of the Dysport wears off. These complications include eyelid and brow ptosis (drooping), vision changes, lip ptosis/asymmetric smile, and difficulty pursing the lips (Kroumpouzos et al., 2021; Lee et al., 2020; Ong & Sherris, 2019; Witmanowski & Błochowiak, 2019).
Eyelid and Eyebrow Drooping
The most common of these are eyelid and/or eyebrow ptosis (drooping). Eyelid ptosis can occur after treatment between the brows, in the forehead, or around the eyes. This can first become visible as early as 2 days after treatment or as late as 10 days after treatment and is more common in those who already experience ptosis or have general weakness in these muscles, such as the elderly.
Some older patients already have ptosis that is not as visible, as their body has compensated by using more of the forehead muscles to help lift the eyelids. Treating the forehead then removes this assistance, causing the eyelid to droop. Loose skin could also contribute to making this more common in older patients. This drooping will recover on its own as the product wears off and is typically much improved after 2 to 4 weeks. In some cases, prescription eye drops can be used for improvement. (Kroumpouzos et al., 2021; Witmanowski & Błochowiak, 2019).
Eyebrow ptosis/drooping can occur after forehead treatment in about 1% to 5% of patients, with an overall drop of about 2.5 mm and difficulty raising the brows (Kroumpouzos et al., 2021). Another brow complication can be the raising out of the outer ends of the eyebrows, which is called a “Mephisto sign” or “Spock brow.” This occurs due to an imbalance of paralyzation, and the likelihood of this occurring can vary depending on individual anatomy and provider experience. If this occurs, the effect can last until the treatment wears off, but additional units can often treat and even out the brow muscles.
Other Potential Side Effects Affecting the Eyes
Other potential eye side effects include diplopia (double vision), ectropion (the eyelid turning outward), blurred vision, dry eyes, watering eyes, and transient strabismus (crossing of the eye that comes and goes). All are quite rare and expected to return to normal as the effects of treatment wears off. Some may require further treatment to keep the eyes safe and moisturized until then.
Lip Drooping
Treatment around the mouth can potentially cause lip ptosis (drooping), an uneven smile, or difficulty pursing the lips. In some cases, treatment of the masseter can cause the opposite of the intended effect, where they become larger to compensate. Treatment of the neck can cause more serious side effects, such as difficulty speaking, swallowing, breathing, and/or hoarseness of the voice. These are also very rare, and the risk can be lowered by limiting the units used (Kroumpouzos et al., 2021; Witmanowski & Błochowiak, 2019).
Dysport and Muscle Atrophy
To start, knowing how the body recovers from Dysport treatment is important. Dysport only inhibits the release of acetylcholine for approximately 3 days. Following this, the body initiates a process where temporary nerve sprouts are generated to the affected muscles, helping to re-establish connections and restore function. Once the original pathways are fully repaired, the temporary nerve sprouts regress (Nawrocki & Cha, 2020). Muscles will indeed atrophy (shrink) when not in use, so the real question is how well and how fully muscles return to normal between treatments or after treatments stop.
The ability of the body to generate nerve sprouts and repair is influenced by a variety of factors. First, treatment with Dysport causes the muscles to produce more of a protein called Insulin-like Growth Factor 1 (IGF-1), which helps to sprout nerves and repair the connections. The amount of IGF-1 in the body is influenced by how much estrogen you have. Younger women have more estrogen and should, therefore, be able to recover more quickly than older women, especially post-menopausal women who are not on hormone replacement therapy.
The creation of Schwann cells is also believed to be boosted after treatment. These cells also assist in nerve regeneration and repair. Individual factors that lower your amount of or ability to create Schwann cells can also delay your recovery. This can include older age, nutritional deficiencies, and certain diseases.
Are Different Muscles Affected in Different Ways?
The speed of repair and amount of nerve sprouting also depends on the type of muscle that is being treated. There are generally three muscle types, type I (slow-twitch), type II-a (fast, fatiguable), and type II-b (fast, fatigue resistant). Type I fibers are believed to repair the fastest. The face has a variety of different muscle types depending on function. For example, the muscles around the eye are mainly type II, while the muscles of the cheek walls are mainly type I (Azizzadeh & Nduka, 2021). The distance of the muscle from the brainstem is also a factor, with closer muscles recovering function more quickly.
All of these factors, along with others such as lifestyle, medical conditions, and sex, should be taken into consideration when making treatment decisions. Atrophy occurs while muscles are inactive, and so treating the muscles before they have fully reestablished neural connections leads to the greatest risk of unwanted atrophy. Using the lowest dose necessary, with proper time to recover between injections, and avoiding too many “touch-up” doses between sessions should allow muscles time to recover between treatments (Salari et al., 2018). Providers should consider individual factors to create personalized plans, and patients should be encouraged to wait for effects to wear off (usually 3-4 months) before seeking retreatment.
Frequently Asked Questions About Neuromodulators
Will I look “frozen” after treatment with Dysport?
You won’t appear ‘frozen’ with Dysport treatment if your provider uses the appropriate amount of units. Our goal is to always give natural-looking results, not completely limit movement to remove every line. Several studies have shown that utilizing the correct dosages with proper retreatment timelines can deliver results without giving a “frozen” look (Ali et al., 2023).
Are there long-term effects of Dysport?
Dysport is widely considered to be a safe treatment for long-term use. There are generally two areas of discussion when it comes to fears of long-term use. The first is muscle atrophy (shrinking) and the second is immunogenicity (developing immunity to treatment). The general fear surrounding muscle atrophy is that after repeated treatment, the muscles will shrink and become weak to the point they do not fully recover or other muscles step in to compensate, changing expressions and function. The concern with immunogenicity is that the body could recognize the Dysport as a foreign substance and develop immunity to it, causing treatment to stop working.
Can I become immune to Dysport?
Immunogenicity (developing immunity) to Dysport does occur, but it is very rare. If treatment is not working, it is first important to ensure that your dosage was high enough and that treatment was performed correctly. Those who do not see an effect of treatment are either classified as primary non-responders (PNR) or secondary non-responders (SNR). PNR patients are those who have never experienced good results, with under a 25% improvement at their first treatment. SNR patients are those who used to experience results but no longer do in at least 2 repeated treatments.
Since Dysport is a foreign substance, the body can develop antibodies to resist it. The body may form antibodies against the core neurotoxin itself or against accessory components of the medication. Not all patients who develop antibodies stop responding, and not all patients who stop responding have antibodies, but they can cause a lack of response. Approximately 3.5% of those who do see response to treatment have antibodies, and 53.5% of patients with SNR have antibodies. Most who develop SNR at first see a loss of effect and slowly progress toward no effect over the next 2 to 3 treatments (Bellows & Jankovic, 2019).
How common is immunity?
As per package inserts, 1.5% of cosmetic Botox (2023) patients developed binding antibodies, while only .19% of cosmetic Dysport (2023) patients developed binding antibodies in trials. Neither company had any patients that developed neutralizing antibodies in trials. Binding antibodies are able to attach to the protein in Dysport or Botox and may or may not have an impact on its effect while neutralizing antibodies would render treatment ineffective.
The reported rate of actual neutralizing antibody formation and complete resistance in cosmetic patients appears to be under 1% (Choudhury et al., 2021). As per Bellows & Jankovic (2019), multiple studies were unable to find any antibody formation in cosmetic patients, including a pooled analysis of 1968 patients over a 13 to 17-month period. It appears that the rate of immunogenicity increases with the amount of product used at each treatment, and it is generally believed that a shorter time between treatments also contributes (Truong et al., 2023; Bellows & Jankovic, 2019).
What happens if a patient develops immunity?
If a patient does develop immunity, there are still options. As noted previously, Xeomin lacks the non-active proteins found in other formulations. Multiple studies have shown that many patients who have developed resistance to other formulations found success with switching to Xeomin (Bellows & Jankovic, 2019). This is likely because many patients have developed antibodies to the non-complexing proteins found in other brands rather than the BoTN itself.
Original formulations of Botox, which contained more complex proteins, led to up to 17% resistance in patients being treated for cervical dystonia, further indicating that this may be the case (Shtefan et al., 2022). A study by Wanitphakdeedecha et al. (2020) found that 30% of non-responders responded to treatment with Xeomin. Antibodies also drop with time, so a long break could allow non-responders to return to treatment in the future (Shtefan et al., 2022).
At Allure Aesthetics, we have only encountered non-responsiveness once. In that particular case, the individual had already developed immunity before seeking treatment with us, and they had a long history of autoimmune issues.
Overall, the concerns about atrophy and immunogenicity lead to the same recommendations—only use as much as you need and wait for the treatment to wear off before being retreated. If these guidelines are followed, the risk should be low, and you should have more natural-looking, cost-effective results.
What should I do before a Botox or Dysport appointment?
Before your appointment, you should let your provider know if any of the following apply to you:
You are taking these medications or supplements:
- You are taking aminoglycoside antibiotics, as these medications also inhibit acetylcholine release, which can compound the effects of your treatment ((Mannina & Nunes, 2020).
- You are taking muscle relaxers, cold medicine, or sleeping medicine, as these may affect the movements of your facial muscles and give your provider an inaccurate perception of the muscles’ strength.
You have the following medical conditions or allergies:
- You have any muscle or nerve condition, such as ALS/Lou Gehrig’s disease, multiple sclerosis, myasthenia gravis, or Lamert-Eaton syndrome, as these can increase the risk of severe side effects from typical doses of Dysport.
- You have a bleeding disorder or are taking medications or substances that affect platelet function, thrombolytics, or anticoagulants, as this will make you more susceptible to bruising, hematomas, or bleeding at the site of injection.
- You have any medical conditions in or around the neck or trouble swallowing, as this could increase your risk of more serious complications.
- You are allergic to albumin and eggs or have had a negative reaction to vaccination in the past, as this could increase your risk of an allergic reaction due to the albumin in Botox. Your provider may recommend an alternative formulation.
- You have a known hypersensitivity to any of the components of the product or have had an unusual reaction to past treatment.
- You are allergic to anesthetic-based topical agents, lidocaine, or other amide-type local anesthetics, as we may use them to numb you for treatment. Your provider may suggest an alternative if you are allergic to any of these medications.
- You have a history of hypertrophic or keloid scarring, as this could potentially occur with any treatment that pierces the skin.
- You are pregnant or breastfeeding, as Dysport has not been tested and proven safe for a fetus or breastfeeding child.
Any of the below applies to you:
- You are planning to have surgery on the face, neck, or chin, as treatments may affect your surgeon’s ability to consult or operate on you.
- You have had other Dysport treatments in the past six months, as that treatment may not have fully worn off.
- You have had other cosmetic treatments or surgeries to the face, neck, or chin, as this will have altered your anatomy and should be taken into consideration.
- You have weak forehead muscles, trouble raising your eyebrows, drooping eyelids, or have had any other abnormal facial changes, as your provider may want to further investigate this first, or at least take it into consideration when determining dosage.
For Dysport pre-care, Allure Aesthetics recommends the following:
- Do not schedule an appointment within 1 to 2 weeks of a special event. It may take up to 2 weeks to see the full effects of treatment. There may be bruising present for a few days after treatment. Ideally, you would not get your first treatment just before a special event, as adjustments may need to be made to find the perfect dose for you.
- Do not schedule any dental appointments, facials, massages, lash/brow appointments, or other procedures for 2 weeks after your appointment. Any pressure on the treated areas could cause a spread of effect.
- Similarly, please note that you cannot wear any tight-fitting masks for 2 weeks.
- Please avoid taking any unnecessary blood-thinning medications, supplements, or foods for 1 week prior to your appointment. This includes, but is not limited to: Advil, Motrin, Ibuprofen, aspirin, almonds/almond milk, fish/sushi/fish oil, garlic, ginger, green tea, alcohol, and workout supplements. All of these medications can cause you to bleed or bruise more during and after your treatment.
- We recommend taking Arnica (unless you are allergic or it is contraindicated with any medications or medical conditions you have, such as high blood pressure) beginning three days before your appointment, which is an herbal medicine that reduces bruising.
What should I do after a Dysport treatment?
For Dysport after-care, Allure Aesthetics recommends:
- Continue avoiding the blood-thinning medications/supplements/food/alcohol listed in our pre-care (unless they are medically necessary) until any bruising and swelling have healed, as these can prolong bruising.
- Avoid exercise and/or hot baths and saunas until bruising and and swelling and healed, as this can increase circulation and prolong the process.
- Avoid tanning or prolonged exposure to sunlight until bruising has healed.
- If you are experiencing any bruising or swelling, continue taking Arnica until it subsides. Follow the instructions in the box carefully, and avoid taking it if you have a medical condition or are on medication that contraindicates its use.
- To avoid spreading the Dysport and the effect to unwanted areas:
- Do not lie or bend over with your head down for at least 3 hours after treatment (squat down instead of bending over at the waist)
- Do not wear any hats, headbands, tight masks, or helmets for at least 24 hours.
- Avoid touching your face or applying makeup for at least 24 hours (Oxygenetix is the only makeup that can be used post-procedure).
- Avoid facials, massages, exfoliating, rubbing/scrubbing, Clarisonic devices, lash/brow appointments, and dental appointments for 2 weeks.
- For masseter, Nefertiti, or marionette line treatments, please avoid rubbing or resting on these areas.
Neuromodulator/Neurotoxin Treatments at Allure Aesthetics
At Allure Aesthetics, located near Philadelphia in King of Prussia, we offer advanced neuromodulator treatments, including Botox, Dysport, and Xeomin. While we provide a range of options, Dysport is our top recommendation, known for delivering faster results, longer-lasting effects, and a naturally refreshed appearance.
Whether you’re seeking to smooth fine lines, enhance confidence, or achieve a more youthful look, our experienced team is here to help you reach your aesthetic goals with personalized, non-surgical treatments.
You can read more information about our Dysport treatments here our learn more here about our medspa in King of Prussia. For more information on how we can tailor them to your goals, please email us at info@allureaestheticsllc.com or book your appointment directly with us here!
References 1
AbbVie. (2023, November). Botox. Prescribing information. http://www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf
Ali, S., AL Bukhari, F., Al Nuaimi, K., Elenany, H., Fakih-Gomez, N., Ghannam, S., Haidar, R., Isse, N., Labib, N., Mosahebi, A., Ravichandran, S., Turkmani, M. G., & Youssef, C. (2023). Consensus statement on the use of botulinum neurotoxin in the Middle East. Clinical, Cosmetic and Investigational Dermatology, 16, 2899–2909. https://doi.org/10.2147/CCID.S420921
Azizzadeh, B., & Nduka, C. (Eds.). (2021). Management of post-facial paralysis with synkinesis. Elsevier.
Bach, K., & Simman, R. (2022). The multispecialty toxin: A literature review of botulinum toxin. Plastic and Reconstructive Surgery – Global Open, 10(4). https://doi.org/10.1097/GOX.0000000000004228
Bellows, S., & Jankovic, J. (2019). Immunogenicity associated with botulinum toxin treatment. Toxins, 11(9). https://doi.org/10.3390/toxins11090491
Bruloy, E., Sinna, R., Grolleau, M. D., Bout-Roumazeilles, A., Berard, E., & Chaput, B. (2019). Botulinum toxin versus placebo: A meta-analysis of prophylactic treatment for migraine. Plastic and Reconstructive Surgery, 143(1). https://doi.org/10.1097/PRS.0000000000005111
Choudhury, S., Baker, M. R., Chatterjee, S., & Kumar, H. (2021). Botulinum toxin: An update on pharmacology and newer products in development. Toxins, 13(1). https://doi.org/10.3390/toxins13010058
Do, T. P., Hvedstrup, J., & Schytz, H. W. (2018). Botulinum toxin: A review of the mode of action in migraine. Acta Neurologica Scandinavica, 137(5), 442–451. https://doi.org/10.1111/ane.12906
Evolus. (2023). Jeuveau. Prescribing information. https://image.newsletter.evolus.com/lib/fe3511737164047c751277/m/1/7a8b57cf-e5b3-4e8f-99ab-6f9ab170cbe5.pdf
References 2
Fernández-Núñez, T., Amghar-Maach, S., & Gay-Escoda, C. (2019). Efficacy of botulinum toxin in the treatment of bruxism: Systematic review. Medicina Oral, Patología Oral y Cirugía Bucal, 24(4), e416–e424. https://doi.org/10.4317/medoral.22923
Gadarowski, M. B., Ghamrawi, R. I., Taylor, S. L., & Feldman, S. R. (2021). PrabotulinumtoxinA-xvfs for the treatment of moderate-to-severe glabellar lines. Annals of Pharmacotherapy, 55(3), 354–361. https://doi.org/10.1177/1060028020943527
Galderma. (2023). Dysport. Prescribing information. Dysport. https://www.dysportusa.com/PI
Guida, S., Farnetani, F., Nisticò, S. P., Mariarosaria, C. G., Babino, G., Pellacani, G., & Fulgione, E. (2018). New trends in botulinum toxin use in dermatology. Dermatology Practical & Conceptual, 8(4), 277–282. https://doi.org/10.5826/dpc.0804a05
Hanna, E., & Pon, K. (2020). Updates on botulinum neurotoxins in dermatology. American Journal of Clinical Dermatology, 21(2), 157–162. https://doi.org/10.1007/s40257-019-00482-2
International Neuromodulation Society. (2018). About neuromodulation. https://www.neuromodulation.com/about-neuromodulation
Kępczyńska, K., & Domitrz, I. (2022). Botulinum toxin—A current place in the treatment of chronic migraine and other primary headaches. Toxins, 14(9). https://doi.org/10.3390/toxins14090619
Kroumpouzos, G., Kassir, M., Gupta, M., Patil, A., & Goldust, M. (2021). Complications of botulinum toxin A: An update review. Journal of Cosmetic Dermatology, 20(6), 1585–1590. https://doi.org/10.1111/jocd.14160
Lee, K. C., Pascal, A. B., Halepas, S., & Koch, A. (2020). What are the most commonly reported complications with cosmetic botulinum toxin type A treatments? Journal of Oral and Maxillofacial Surgery, 78(7), e1–-e9. https://doi.org/10.1016/j.joms.2020.02.016
References 3
Lewandowski, M., Świerczewska, Z., & Barańska-Rybak, W. (2022). Off-label use of botulinum toxin in dermatology—Current state of the art. Molecules, 27(10). https://doi.org/10.3390/molecules27103143
Mannina, C. G., & Nunes, E. L. (2020). Drug interaction with botulinum toxin. Journal of Oral Care and Dentistry, 2(1), 1–6. https://doi.org/10.5281/zenodo.3937965
Merz. (2023, September). Xeomin. Prescribing information. https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=3f35d6e0-3450-4abc-a0da-cc7b277e7c6e&type=pdf
Nawrocki, S., & Cha, J. (2020). Botulinum toxin: Pharmacology and injectable administration for the treatment of primary hyperhidrosis. Journal of the American Academy of Dermatology, 82(4), 969–979. https://doi.org/10.1016/j.jaad.2019.11.042
Ong, A. A., & Sherris, D. A. (2019). Neurotoxins. Facial Plastic Surgery, 35(03), 230–238. https://doi.org/10.1055/s-0039-1688844
Park, J.-Y., Sunga, O., Wanitphakdeedecha, R., & Frevert, J. (2020). Neurotoxin impurities: A review of threats to efficacy. Plastic and Reconstructive Surgery – Global Open, 8(1). https://doi.org/10.1097/GOX.0000000000002627
References 4
Park, M. Y., & Ahn, K. Y. (2021). Scientific review of the aesthetic uses of botulinum toxin type A. Archives of Craniofacial Surgery, 22(1), 1–10. https://doi.org/10.7181/acfs.2021.00003
Salame, Eber, Ariel E., & Dover, Jeffrey. (2023). DaxibotulinumtoxinA-lanm (DaxxifyTM): A comprehensive overview. Skin Therapy Letter, 28(4), 1–3.
Salame, N., Cox, S. E., & Dover, J. S. (2024). Daxxify: Recommendations for treatment: How and when to change a patient from one neurotoxin to another. Advances in Cosmetic Surgery, 7(1), 9–23. https://doi.org/10.1016/j.yacs.2023.12.001
Salari, M., Sharma, S., & Jog, M. S. (2018). Botulinum toxin induced atrophy: An uncharted territory. Toxins, 10(8). https://doi.org/10.3390/toxins10080313
Schulze, J., Neumann, I., Magid, M., Finzi, E., Sinke, C., Wollmer, M. A., & Krüger, T. H. C. (2021). Botulinum toxin for the management of depression: An updated review of the evidence and meta-analysis. Journal of Psychiatric Research, 135, 332–340. https://doi.org/10.1016/j.jpsychires.2021.01.016
Shtefan, V., Fletcher, J., & Duclos, O. A. (2022). Causes of botulinum toxin treatment failure. Clinical, Cosmetic and Investigational Dermatology, 15, 1045–1049. https://doi.org/10.2147/CCID.S363321
References 5
Solish, N., Carruthers, J., Kaufman, J., Rubio, R. G., Gross, T. M., & Gallagher, C. J. (2021). Overview of daxibotulinumtoxinA for injection: A novel formulation of botulinum toxin type A. Drugs, 81(18), 2091–2101. https://doi.org/10.1007/s40265-021-01631-w
Solstice Neurosciences. (2019). Prescribing information. Myobloc. https://www.myobloc.com/files/Myobloc-Prescribing-Information.pdf
The American Society of Plastic Surgeons. (2023). 2022 ASPS procedural statistics release. https://www.plasticsurgery.org/documents/news/Statistics/2022/plastic-surgery-statistics-report-2022.pdf
Truong, D., Dressler, D., Hallett, M., Zachary, C., & Pathak, M. (2023). Manual of botulinum toxin therapy (3rd ed.). Cambridge University Press.
Wanitphakdeedecha, R., Kantaviro, W., Suphatsathienkul, P., Tantrapornpong, P., Yan, C., Apinumtham, C., & Srinoulprasert, Y. (2020). Association between secondary botulinum toxin A treatment failure in cosmetic indication and anti-complexing protein antibody production. Dermatology and Therapy, 10(4), 707–720. https://doi.org/10.1007/s13555-020-00397-5
Witmanowski, H., & Błochowiak, K. (2019). The whole truth about botulinum toxin – A review. Advances in Dermatology and Allergology/Postępy Dermatologii i Alergologii, 37(6), 853–861. https://doi.org/10.5114/ada.2019.82795
Yi, K.-H., Lee, J.-H., Kim, S.-O., Hu, H., Lee, H.-J., Choi, Y.-J., Ahn, T.-H., & Kim, H.-J. (2023). Botulinum neurotoxin injection for treating plunged nose and post-rhinoplasty: Anatomical perspectives of depressor septi nasi, nasalis, leveator labii superioris alaeque nasi muscle. Anatomy & Cell Biology, 56(4), 409–414. https://doi.org/10.5115/acb.23.054






